Galvanic corrosion is the degradation of materials produced by an external electrochemical attack when they come into contact with each other,
This effect occurs when 2 dissimilar materials come into contact with each other. Conditions such as water or environmental humidity act as an electrolyte, causing an electrochemical reaction called corrosion. This corrosion is progressive, causing the materials to deteriorate, giving rise to a loss of functionality in the fixing, as well as aesthetic problems. The presence of traces of salt in coastal areas and marine environments accelerates the corrosion process.
One way to minimise the impact is using materials with electrochemical potentials as similar as possible in contact with each other, so that the decreased potential difference between them minimises the effect of contact corrosion.
The following table shows, by way of example, the potential of various metals in contact with seawater; the nobler the material used, the less active it is:
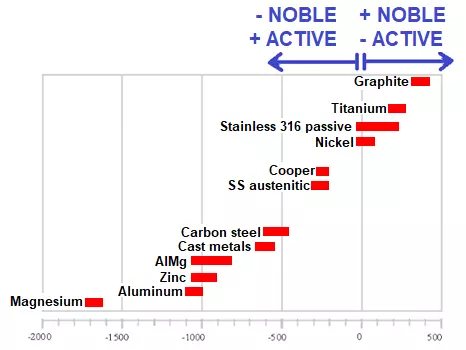
Electrochemical series in sea water
Therefore, joints with materials that have very similar electrolytic potentials reduce the possibility of galvanic contact corrosion. However, in joints with materials that have very different electrochemical potentials, deterioration will occur, preferentially in the one with lower potential (less noble; more active).

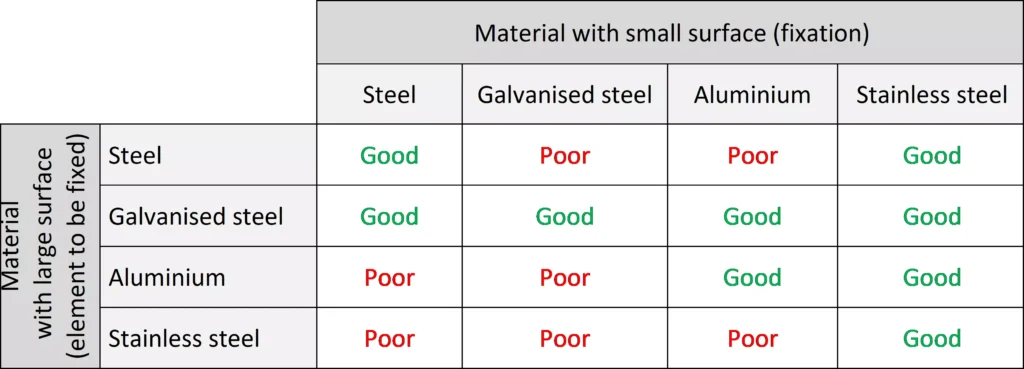
Generally speaking, we will use an anchor (material with a small surface area) with the same or greater electrochemical potential as the piece to be fixed (material with a large surface area), so that the anchor is more noble and therefore less active from an electrochemical point of view, so that the smaller surface behaves better against corrosion and does not lead to failure caused by this type of corrosion in the fixing.
The following table shows the type of material depending on the contact surface, establishing different combinations between materials that allow us to select the right material for our fixing depending on the element that we want to fix, considering the effect of galvanic corrosion according to the surface and the material:
Ultima revisione: FAQ23 rev0

